FDA approves new drug that may help stop and even reverse a rare, fatal condition that doctors call a ‘ticking time bomb’ | CNN
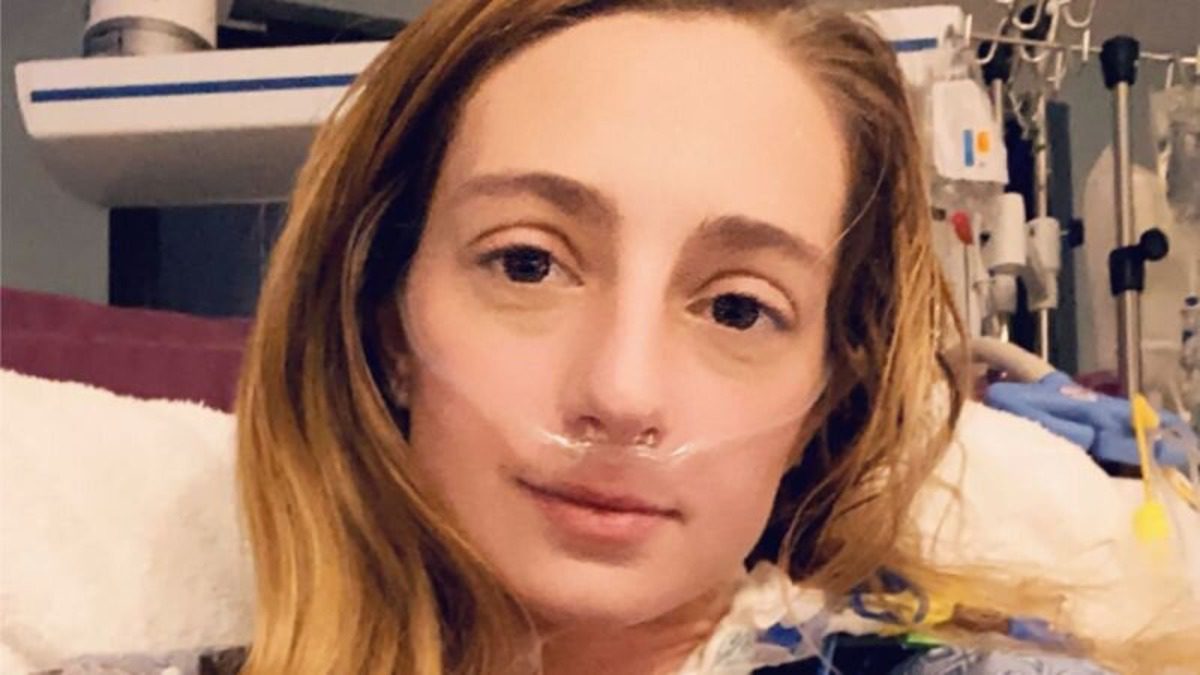
The article discusses the approval of a new medication, Winrevair, by the FDA to treat pulmonary arterial hypertension (PAH), a rare and fatal condition. It recounts the story of Katrina Barry, who was diagnosed with PAH at a young age and participated in clinical trials for the drug. Winrevair, manufactured by Merck, works by targeting proteins that contribute to PAH, potentially reversing the disease’s progression. Clinical trials showed promising results, including improved walking distance for patients. Despite potential side effects like bleeding and dizziness, the drug offers hope for PAH patients, with some experiencing significant improvements and even discontinuing other therapies. However, uncertainties remain regarding its long-term effectiveness and whether it will replace existing treatments. The drug’s expected cost, around $243,000 annually per patient, raises concerns about accessibility. Nonetheless, clinicians express optimism about its potential to transform PAH treatment and provide newfound hope for patients facing this challenging condition.
#PAHtreatment #Winrevair #MedicalBreakthrough #HopeforPAH #HealthcareInnovation