Fujifilm Receives 510(k) Clearance for CAD EYE®, New AI-Powered Endoscopic Imaging Technology for Colonic Polyp
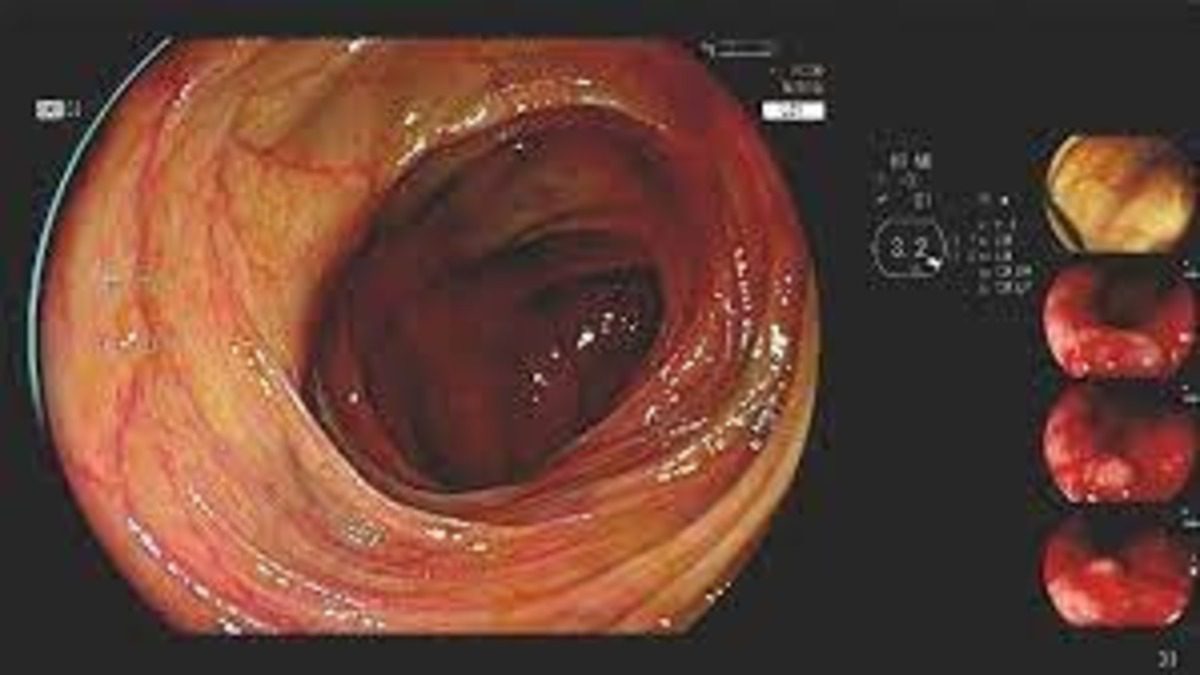
Fujifilm has secured FDA clearance for its CAD Eye AI-powered system, enhancing endoscopic imaging. This system aids in real-time detection of colonic mucosal lesions during colonoscopy, assisting in the identification and removal of pre-cancerous lesions. It competes with Medtronic’s GI Genius system, offering features like compatibility with white light imaging and Linked Color Imaging (LCI). Fujifilm’s system stands out with an enhanced visualization mode and seamless integration into physicians’ workflows. Developed with deep learning technology, CAD Eye has been validated using histologically confirmed polyps, demonstrating improved detection rates without increasing procedure time. Clinical studies indicate its superiority over conventional colonoscopy, detecting more adenomas and neoplastic lesions. Dr. Sravanthi Parasa of the Swedish Medical Center applauds this advancement, highlighting its potential to enhance patient outcomes and reduce the risk of missed lesions, thereby advancing gastrointestinal healthcare. Fujifilm plans to release CAD Eye commercially after completing a limited market evaluation, aiming to improve the quality of colonoscopies and bolstering their commitment to technological innovation in healthcare.
#Endoscopy #AI #HealthcareInnovation #Colonoscopy #PatientCare Hashtags: #Fujifilm #CADEye #GastrointestinalHealth #MedicalTechnology #CancerDetection